Our recent activity
Agomab Announces $89 Million Series D Financing to Support Broad Fibrosis-focused Pipeline
READ THIS NEWS IN SPANISH
Round includes participation from new investors Sanofi and Invus, as well as existing investors
Proceeds will fund clinical development of lead candidate AGMB-129 in fibrostenosing Crohn’s disease, AGMB-447 in idiopathic pulmonary fibrosis and AGMB-101 in liver cirrhosis
Agomab Therapeutics NV (‘Agomab’) today announced a $89 million (€82.1 million) Series D financing round, with participation from new investors Sanofi and Invus, as well as existing investors.
The proceeds from the Series D will be used to further advance the ongoing clinical development of Agomab’s lead candidate, AGMB-129, a gut-restricted oral small molecule inhibitor of ALK5 (TGFβ1R), in patients with fibrostenosing Crohn’s disease (FSCD). Interim data from the Phase 2a STENOVA trial are expected in the first quarter of 2025. In addition, proceeds will be used to advance the clinical development for AGMB-447, a lung-restricted inhaled small molecule inhibitor of ALK5, that is currently in a Phase 1 clinical trial in healthy subjects and patients with idiopathic pulmonary fibrosis (IPF), as well as initial clinical development of AGMB-101, a full MET agonistic antibody currently in the final stages of IND-enabling studies, which the company intends to develop for liver cirrhosis.
“We are thrilled to have Sanofi and Invus joining our investor syndicate. Their investment as well as the continued support from our existing shareholders is another validation of the trailblazing work our team is conducting in the fields of fibrostenosing Crohn’s disease and idiopathic pulmonary fibrosis.” said Tim Knotnerus, Chief Executive Officer at Agomab Therapeutics. “Through this financing round we will create further optionality and the capital will allow us to accelerate our efforts in developing our novel potential therapies.”
About Agomab
Agomab is focused on achieving disease modification by modulating fibrosis and regeneration in chronic indications such as fibrostenosing Crohn’s disease and idiopathic pulmonary fibrosis. We do this by targeting biologically validated pathways – including Transforming Growth Factor β and Hepatocyte Growth Factor – and by applying specialized capabilities in organ-restricted small molecules and high affinity antibodies. With a differentiated clinical pipeline across several fibrotic disorders, end-to-end research and development capabilities, a proven BD track-record and a strong investor base, Agomab is building a leading biopharma company.

 Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
 AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
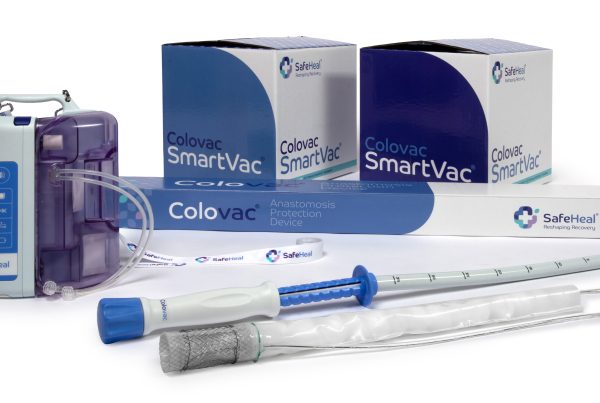 Asabys Partners Leads €10 Million Extension of SafeHeal’s Oversubscribed Series C Financing to Accelerate Commercialization of Colovac®
Asabys Partners Leads €10 Million Extension of SafeHeal’s Oversubscribed Series C Financing to Accelerate Commercialization of Colovac®


