Our recent activity
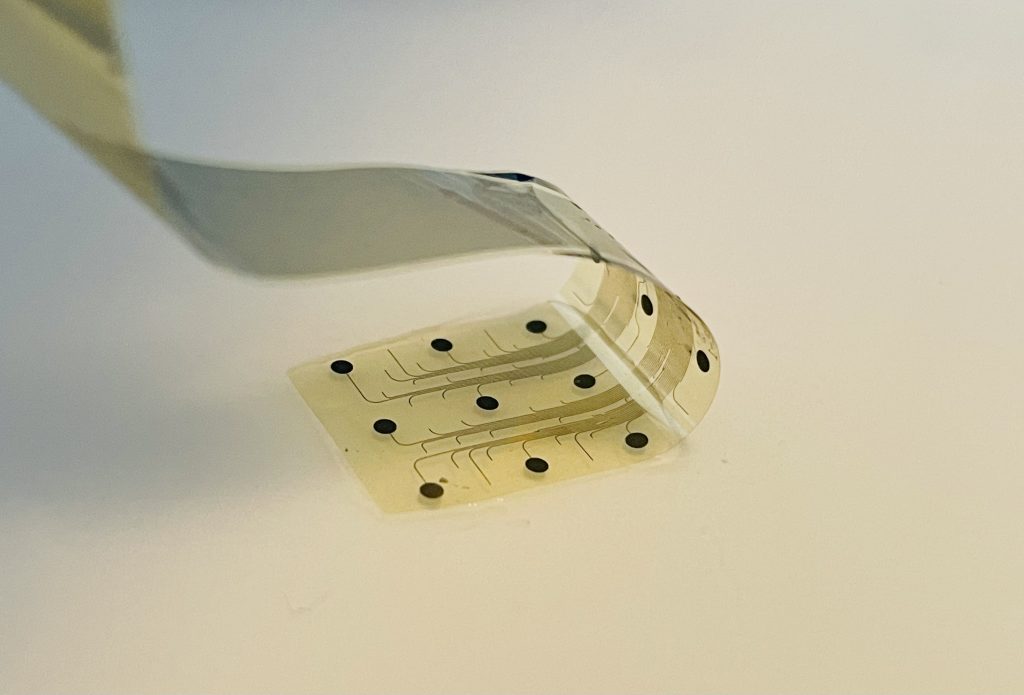
INBRAIN Neuroelectronics announces FDA Breakthrough Device designation for its graphene-based intelligent network modulation platform
A first-of-a-kind device, the INBRAIN Intelligent Network Modulation System is designed to offer a new treatment option for patients with Parkinson’s disease.
It harnesses the properties of graphene and the power of machine learning software to provide highly targeted and adaptive neuroelectronic therapy.
INBRAIN Neuroelectronics is a health-tech company that originated as a spin-off of the Catalan Institute of Nanoscience and Nanotechnology (ICN2) and ICREA, with the participation of the IMB-CNM-CSIC.
INBRAIN Neuroelectronics S.L., a health-tech company dedicated to developing the world’s first smart graphene-neural platform, today announced that its Intelligent Network Modulation System has been granted the Breakthrough Device Designation (BDD) from the U.S. Food & Drug Administration (FDA) as an adjunctive therapy for treating Parkinson’s disease. INBRAIN Neuroelectronics is a spin-off company originated from the Catalan Institute of Nanoscience and Nanotechnology –specifically, the Advanced Electronic Materials and Devices Group led by Prof. José Antonio Garrido– and ICREA, with the participation of the IMB-CNM-CSIC.
The INBRAIN system harnesses the power of graphene, a two-dimensional material made of a lattice of carbon atoms only one atom thick. The thinnest material known, yet stronger than steel, graphene exhibits a unique combination of electrical and mechanical properties that makes it ideal for neurotechnology innovation. INBRAIN’s neural platform technology enables ultra-high signal resolution at levels never achieved before and uses machine learning software that decodes therapy-specific biomarkers. This information is exploited to provide highly targeted and adaptive neuroelectronic treatments aimed at rebalancing pathological neural networks.
“This Breakthrough Device Designation from the FDA signifies the potential of the INBRAIN neural platform to further improve the lives of patients with Parkinson’s disease,” commented Dan Gnansia, INBRAIN Neuroelectronics Head of Clinical Affairs. “We look forward to working with the agency to help bring this important advance into clinical practice.”
The BDD recognizes devices that represent a major innovation or offer reasonable evidence of significant advantage over existing approved or cleared alternatives. It also expedites the development and FDA review of new devices with the potential to more effectively treat or diagnose life-threatening or irreversibly debilitating conditions.
“From the current available clinical brain interfaces, deep brain stimulation (DBS) is a successful therapy to treat Parkinson’s disease. However, current leads have restrictions regarding their relatively large size and low density, limiting their precision for targeting of small deep structures such as the subthalamic nucleus (STN), and the spatial or signal resolution when sensing the local brain electrical activity,” explained Helen Bronte Stewart, professor of neurology and neurological sciences at Stanford
University School of Medicine. “INBRAIN’s new generation of ultrathin graphene-based high resolution interfaces and associated network platform may vastly improve the precision, efficiency and efficacy of DBS and closed-loop or adaptive modulation. The FDA Breakthrough Device designation is a statement to how this technology may be a paradigm shift in the scope of neuromodulation for people with Parkinson’s disease and hopefully for other neuropsychiatric conditions in the future.”
Thanks to the FDA Breakthrough Device recognition of the INBRAIN system, the company will receive feedback throughout the regulatory process, as well as prioritized review by the agency.
“INBRAIN is dedicated to leveraging new discoveries in materials science and transforming them into safe and effective breakthrough therapy applications,” added Carolina Aguilar, INBRAIN Neuroelectronics CEO and co-founder “We anticipate developing our technology to treat other conditions affecting the central and peripheral nervous systems to make BCI technology relevant in neuro and bioelectronics.”
About INBRAIN Neuroelectronics
INBRAIN Neuroelectronics S.L., founded in 2020, is a health-tech company dedicated to developing the world’s first intelligent graphene-neural platform to treat a variety of conditions. INBRAIN develops semiconductors derived brain computer interface (BCI) technology that applied in neuromodulation is designed to decode and modulate brain activity in high resolution, using artificial intelligence to trigger adaptive responses for personalized neurological treatment in Parkinson’s disease, epilepsy and speech impairment. Among its founders are ICREA Prof. Jose A. Garrido, group leader at the ICN2 (Barcelona, Spain), Prof. Kostas Kostarelos, group leader at the ICN2 and at the University of Manchester (UK), and Dr Anton Guimerà, staff researcher at the IMB-CNM-CSIC (Barcelona, Spain).
For more information, please visit www.inbrain-neuroelectronics.com, or find us on LinkedIn.
About INNERVIA Bioelectronics
INNERVIA Bioelectronics is a fully owned subsidiary of INBRAIN, dedicated to using graphene technology to develop an intelligent neural platform for peripheral nerve applications in collaboration with Merck KGaA (Darmstadt, Germany) in areas of Merck’s therapeutic interest.
Disclaimer: The INBRAIN Intelligent Network Modulation System is limited by Federal (or United States) law to investigational use only.
For more information:
Institut Català de Nanociència i Nanotecnologia (ICN2) Marketing and Communication Department Àlex Argemí, Head of Marketing and Communication [email protected]; +34 635 861 543
Dr Virginia Greco, Science Writer and Communication Officer [email protected]
About the ICN2
The Institut Català de Nanociència i Nanotecnologia (ICN2) is dedicated to the development of knowledge, materials and devices in the wide field of health, energy, environment and the technologies of computers and communications. Its experience is in the nanoscale where new properties and interactions, as well as ways of using them in the daily life, are constantly being discovered. Amongst its goals are reuniting scientific personnel with different competences in the look for a better science, a better teaching and a higher impact on society, at the same time it explores new ways of interacting with local and global industries.
The institute was credited as a Centre d’Excel·lència Severo Ochoa in 2014 and the Ministerio de Ciencia, Innovación y Universidades renovated this prize in 2018 and 2023. Amongst its patrons are the Generalitat de Catalunya, the Consejo Superior de Investigaciones Científicas (CSIC) and the Universitat Autònoma de Barcelona (UAB), where the institute is placed. ICN2 is a CERCA centre and also one of the founding members of the Barcelona Institute of Science and Technology (BIST) and the European Graphene Flagship.
 Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
 AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
 Asabys Partners Leads €10 Million Extension of SafeHeal’s Oversubscribed Series C Financing to Accelerate Commercialization of Colovac®
Asabys Partners Leads €10 Million Extension of SafeHeal’s Oversubscribed Series C Financing to Accelerate Commercialization of Colovac®


