Our recent activity
Medasense selected as one of “Fierce 15 Medtech Companies of 2022”
As the opioid epidemic exacerbates the complex field of pain management, Israel’s Medasense Biometrics is on a mission to quantify and personalize patient suffering.
At the heart of that quest is the company’s artificial-intelligence-powered patient monitor designed to track nociception, or the physiological response to pain. Dubbed PMD-200, the monitor comes equipped with Medasense’s own alchemy of nociception level (NOL) technology designed to objectively quantify a patients pain response, in turn allowing healthcare professionals to provide bespoke pain treatment.
What makes Medasense fierce: Medasense won FDA marketing authorization for its technology in February through its de novo premarket review pathway.
The technology has been leveraged for much longer in Europe, where PMD-200 scored a CE mark back in 2017. There, Medasense’s platform is largely distributed by Medtronic, which covers deals in the U.K., France, Spain and Portugal, Italy, Greece, Germany, Austria, Switzerland and various Nordic countries.

 Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
Ona Therapeutics, a portfolio company of Asabys Partners and Alta Life Sciences, appoints Dr. Antoine Yver as Chair of the Board to accelerate its ADC programs towards clinical development
 AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
AltamarCAM and Asabys agree to integrate Aliath, their life sciences unit, into Asabys
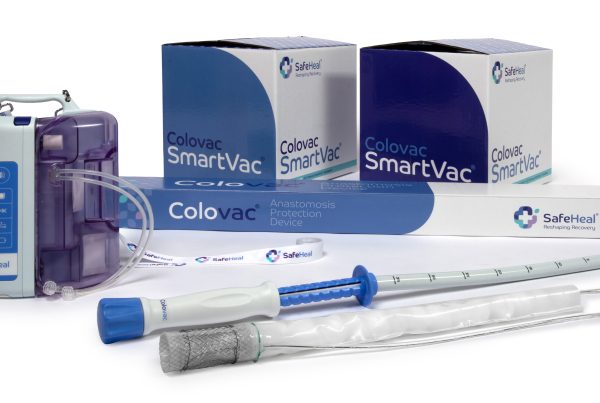 Asabys Partners Leads €10 Million Extension of SafeHeal’s Oversubscribed Series C Financing to Accelerate Commercialization of Colovac®
Asabys Partners Leads €10 Million Extension of SafeHeal’s Oversubscribed Series C Financing to Accelerate Commercialization of Colovac®


